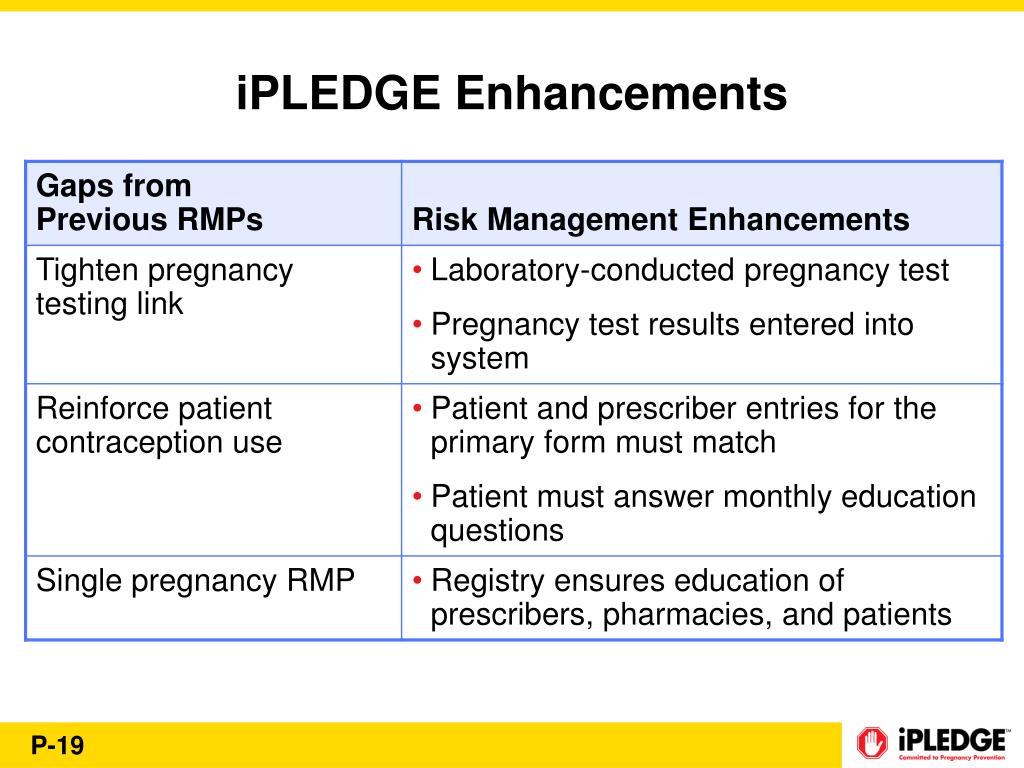
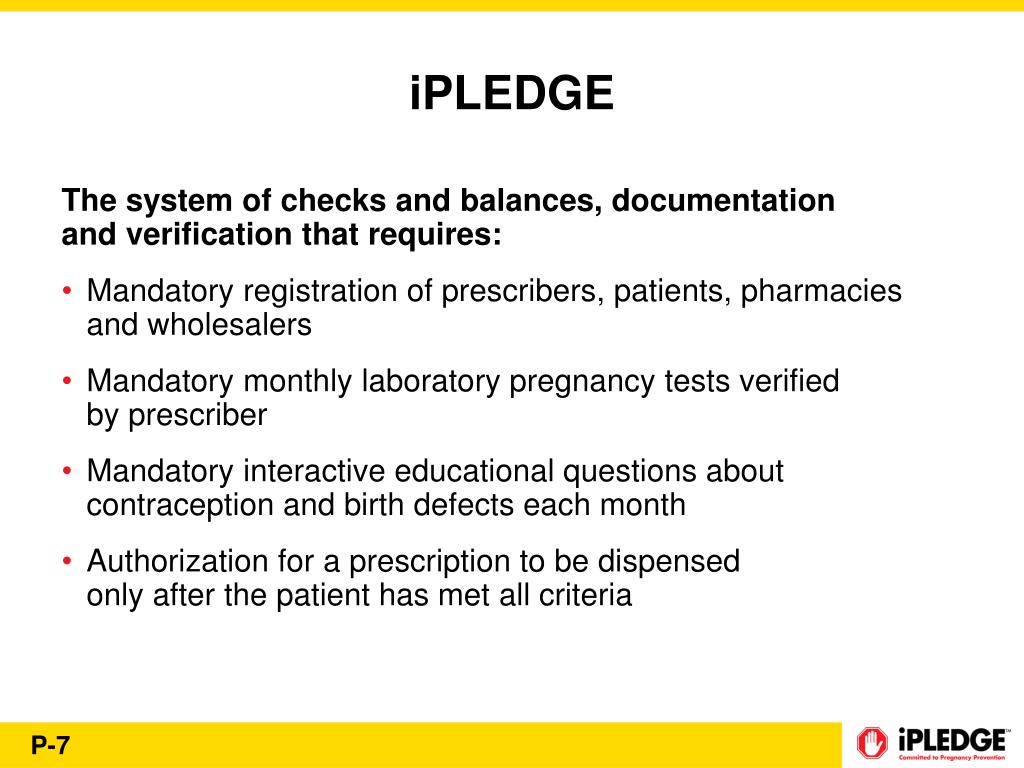
Ipledge Rems Fact Sheet - This system is for the use of authorized users only. The rems is required by the u.s. Food and drug administration (fda) to. Individuals using this computer system without authority, or in excess of their authority, are subject to having all of their activities on. The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure.
Food and drug administration (fda) to. The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. The rems is required by the u.s. Individuals using this computer system without authority, or in excess of their authority, are subject to having all of their activities on. This system is for the use of authorized users only. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure.
Individuals using this computer system without authority, or in excess of their authority, are subject to having all of their activities on. The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. The rems is required by the u.s. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. Food and drug administration (fda) to. This system is for the use of authorized users only.
BLENREP REMS Fact Sheet
The rems is required by the u.s. The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. Food and drug administration (fda) to. Individuals.
Petition · Cancel iPLEDGE REMS Program so that isotretinoin patients
Food and drug administration (fda) to. Individuals using this computer system without authority, or in excess of their authority, are subject to having all of their activities on. The rems is required by the u.s. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. This system is for the use.
Isotretinoin (Accutane) and iPledge Program Patient Information YouTube
The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. This system is for the use of authorized users only. Food and drug administration (fda) to. The rems is required by the u.s. Individuals using this computer system without authority, or in excess.
PPT Isotretinoin Pregnancy Risk Management Program PowerPoint
The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. Food and drug administration (fda) to. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. This system is for the use of authorized.
Accutane Ipledge Registration
Individuals using this computer system without authority, or in excess of their authority, are subject to having all of their activities on. The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. The rems is required by the u.s. This system is for.
iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) FDA
The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. This system is for the use of authorized users only. Individuals using this computer system without authority, or in excess of their authority, are subject to having all of their activities on. The ipledge program rems (risk evaluation and mitigation strategy).
Guide To IPLEDGE PDF Birth Control Human Reproduction
The rems is required by the u.s. Food and drug administration (fda) to. This system is for the use of authorized users only. The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. Individuals using this computer system without authority, or in excess.
iPLEDGE allows athome pregnancy tests during pandemic The Hospitalist
The rems is required by the u.s. Food and drug administration (fda) to. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. This system is for the use of authorized users only. Individuals using this computer system without authority, or in excess of their authority, are subject to having all.
PPT Isotretinoin Pregnancy Risk Management Program PowerPoint
The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. This system is for the use of authorized users only. Food and drug administration.
Pregnancy prevention programs for medications used in dermatology
The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. Food and drug administration (fda) to. Individuals using this computer system without authority, or.
This System Is For The Use Of Authorized Users Only.
The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. The rems is required by the u.s. The ipledge program rems (risk evaluation and mitigation strategy) the ipledge program rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize. Food and drug administration (fda) to.