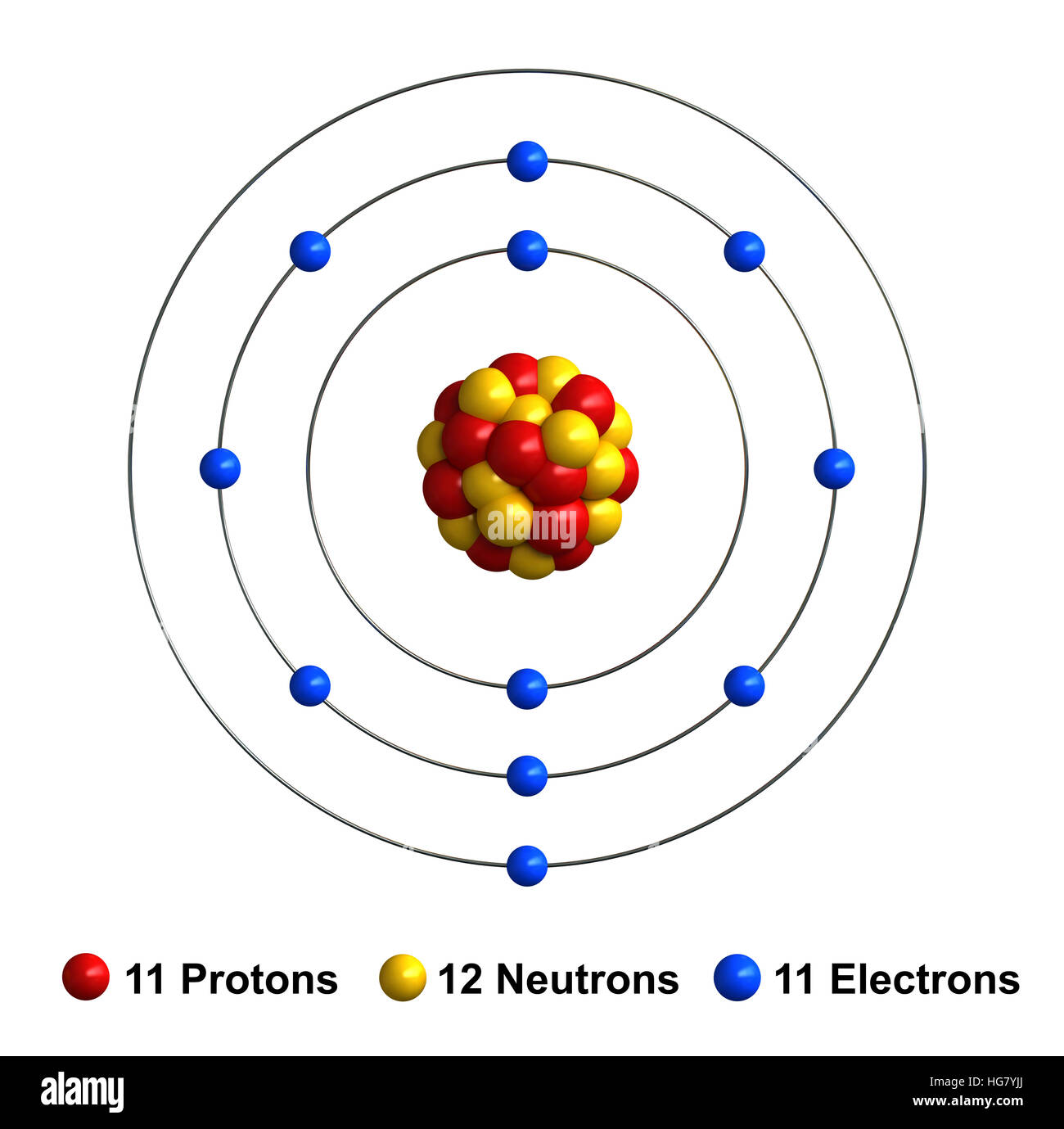
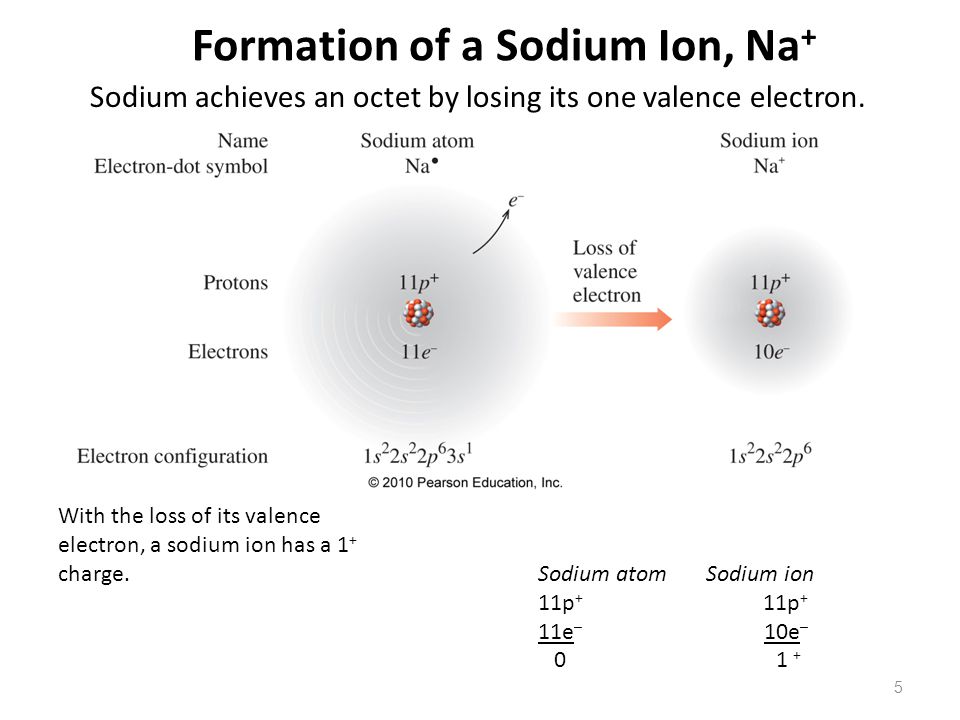
To Form An Ion A Sodium Atom - (i) formation of sodium ion: A neutral sodium atom consists of 11 protons and 11 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When sodium loses its outermost electron, the atom transforms. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative.
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge.
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A neutral sodium atom consists of 11 protons and 11 electrons. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. (i) formation of sodium ion:
Solved Two students worked together to create a bohr model of a sodium
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose.
Draw a diagram to show the electronic structure of a sodium Quizlet
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. The sodium ion, na +, has the electron configuration with an octet of electrons. (i) formation of sodium ion: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative.
Structure Of Sodium Atom Hot Sex Picture
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: A neutral sodium atom consists of 11 protons and 11 electrons. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ions form when atoms lose or gain electrons close.
Sodium Ion Bohr Model
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge..
Sodium Atom Bohr Model Vector Illustration 267662272
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: When sodium loses its outermost electron, the atom transforms. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ions form when atoms lose or gain electrons close electron subatomic.
Formation+of+a+Sodium+Ion,+Na+
(i) formation of sodium ion: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When sodium loses its outermost.
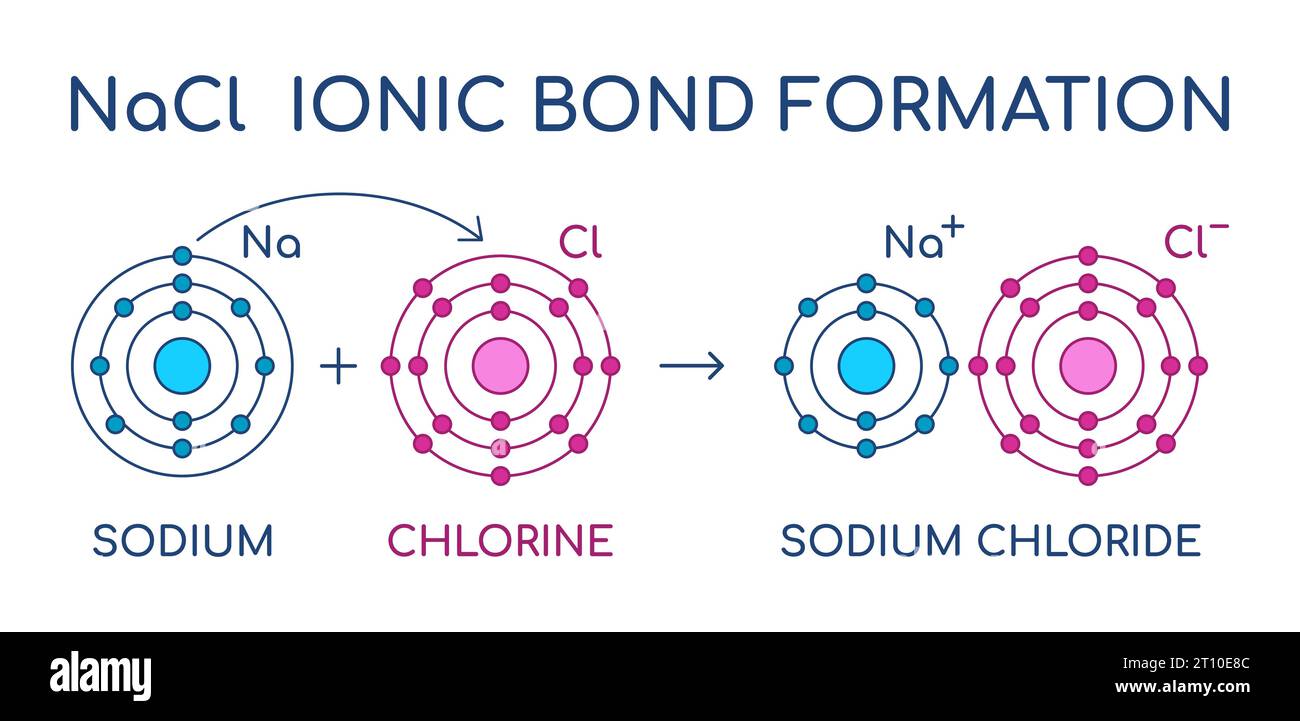
Sodium Chloride ionic bond formation. NaCl structure. Sodium and
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. The sodium ion, na +, has the electron configuration with an octet of electrons. (i) formation of sodium ion: When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or.
How To Draw A Sodium Ion Image to u
The sodium ion, na +, has the electron configuration with an octet of electrons. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or.
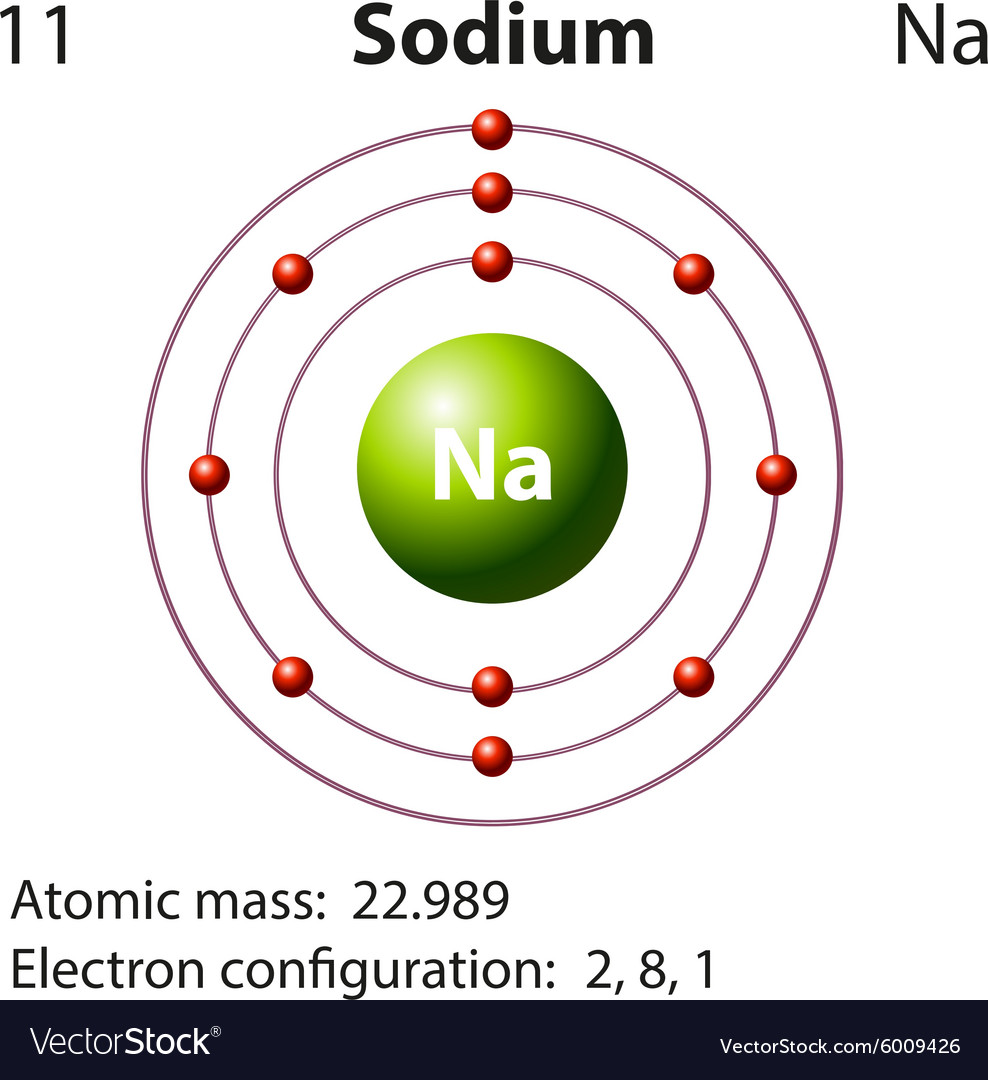
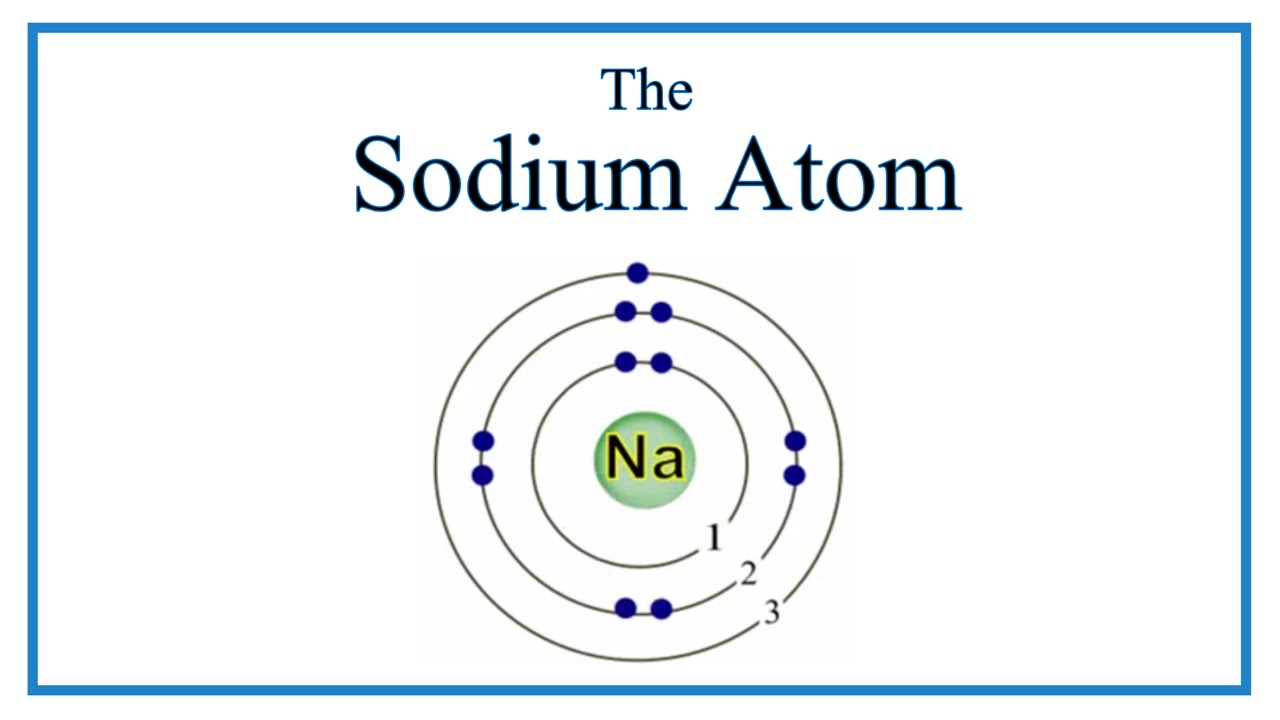
The structure of the sodium atom is shown in the figure given alongside
A neutral sodium atom consists of 11 protons and 11 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. (i) formation of sodium ion: Ions form when atoms lose or gain electrons close.
When A Sodium Atom Reacts With A Chlorine Atom To Form A Compound The
A neutral sodium atom consists of 11 protons and 11 electrons. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge..
Ions Form When Atoms Lose Or Gain Electrons Close Electron Subatomic Particle, With A Negative.
(i) formation of sodium ion: When sodium loses its outermost electron, the atom transforms. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative.
When Sodium Atoms Form Ions, They Always Form A 1+ Charge, Never A 2+ Or 3+ Or Even 1− Charge.
A neutral sodium atom consists of 11 protons and 11 electrons.